Cell Connections
John Bissler, MD, Le Bonheur chief of Pediatric Nephrology and director of the Tuberous Sclerosis Center of Excellence, recently published research in Biology investigating the cellular-level dynamics that lead to polycystic kidney disease (PKD) when the Pkd1 gene or the Tsc2 gene is deleted.
The results showed that deletions of either gene increased the production of extracellular vesicles (EVs), particles that provide communication between cells, which appear to recruit genetically unaltered cells to participate in the development of kidney cysts. In addition, deletion of either the Pkd1 or Tsc2 gene caused kidney cells to take up more EVs and hold onto the EVs for a longer period of time. Researchers hope that further understanding of EVs’ impact on PKD will provide an avenue for new therapies.
In a recent publication, Le Bonheur Chief of Pediatric Nephrology and Director of the Tuberous Sclerosis Center of Excellence John Bissler, MD, explored how deletion of the Pkd1 or Tsc2 genes leads to polycystic kidney disease at a cellular level.
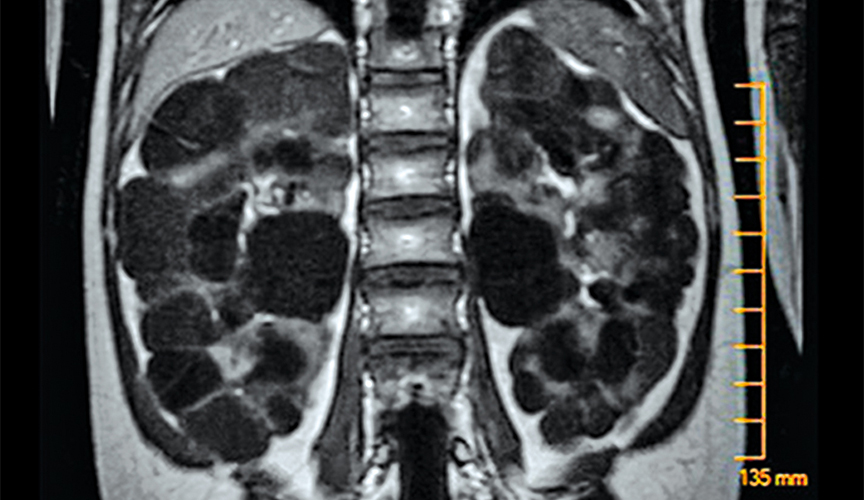
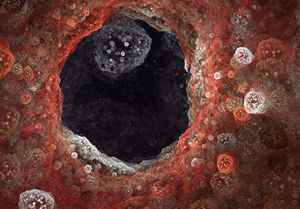
Polycystic kidney disease (PKD) causes cysts to form all over the kidneys (pictured above), leading to high blood pressure and consistently decreasing kidney function. Bissler hopes that the results of this study can lead to new therapies for patients with PKD.
“Patients with PKD and tuberous sclerosis complex (TSC) are born with typical kidneys but quickly develop cysts causing a premature loss of kidney function,” said Bissler. “The more we understand how EVs are involved in the development of PKD, the more potential we have for early intervention to preserve kidney function.”
Results also showed that primary cilia, structures on the surface of cells that act as antennae or sensors, play a large role in transmitting and receiving EVs. When researchers deleted the Pkd1 gene and the gene responsible for the protein that builds cilia, EV production was lowered and PKD was less severe.
Other results included:
Cells without the Pkd1 gene produced more EVs than cells without the Pkd2 gene.
Previously published studies from Bissler’s group showed that Tsc2 gene deletion greatly increased EV production compared to Tsc1 deletion. The same proved to be true when comparing Pkd1 and Pkd2 deletion — with the former producing twice as many EVs.
In addition, EVs from cells without Pkd2 had changes on the surface, which raised the possibility that they may interact or bind differently with cells.
Removing primary cilia lowers EV production.
Researchers added a treatment to kidney cells to remove primary cilia. This caused a significant reduction in EVs, showing that primary cilia are important contributors to the production of EVs.
Deletion of the Tsc2 gene leads to EVs that migrate to the kidney at high rates.
To understand how EVs move through the body, researchers used a mouse model to isolate EVs from kidney cysts and inject them into the peritoneal cavity. Mice without a functioning Tsc2 gene had EVs that migrated to the kidney at a significantly greater amount than those with the Tsc2 gene. EVs from cells with mutations in Pkd1 or Pkd2 also had significantly increased migration to the kidneys.
Researchers posited that kidney cyst EVs must have a method to conscript cells to migrate to the kidneys.
Gene deletions in Pkd1, Pkd2, Tsc1 and Tsc2 caused cells to take in EVs faster and hold them longer.
EVs from cells with Pkd1 deletion had an uptake process 14 times faster and clearance five times slower than those from the cells with Pkd2 deleted. These results suggest that polycystin-1, a protein produced by the Pkd1 gene, regulates EV trafficking.
All of these results provide insight into the cellular-level mechanisms that lead to PKD, particularly the role that EVs play in this process.
“Our study suggests the possibility that EV production rates, tissue half-life, target homing and cargo differences may be involved in the disease process,” said Bissler. “Additional studies can help us identify diagnostic and prognostic biomarkers and ultimately reveal novel therapies to reduce cystic disease.”
Help us provide the best care for kids.
Le Bonheur Children's Hospital depends on the generosity of friends like you to help us serve 250,000 children each year, regardless of their family’s ability to pay. Every gift helps us improve the lives of children.
Donate Now