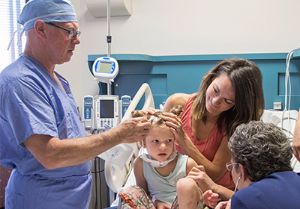
Fenfluramine slows seizure activity in patients
Le Bonheur Children’s neurologists recently began enrolling patients in a new clinical trial of fenfluramine, which aims to help minimize the frequency of seizures for children with Dravet Syndrome.
The trial is in its second phase, and Le Bonheur is one of only four children’s hospitals in the region to offer the fenfluramine drug trial.
Early results have found that patients who use fenfluramine have had fewer seizures, says Tracee Ridley-Pryor, MSN, RN, CCRC, the Neuroscience Institute’s lead clinical research coordinator. Patients on the medication also have shown attention, behavior, learning comprehension and language development improvements.
“The efficacy of fenfluramine has been demonstrated in the number of seizure-free days our patients have experienced while on the investigational medication, up to 29 and 31 seizure-free days, respectively,” Ridley-Pryor said. “For these families, this has been the greatest period of time their child has gone without a seizure since receiving a diagnosis of epilepsy.”
Kevin and Katie Peters-Larson’s 3-year-old daughter, Maelee, is one of the children enrolled in the fenfluramine drug trial.
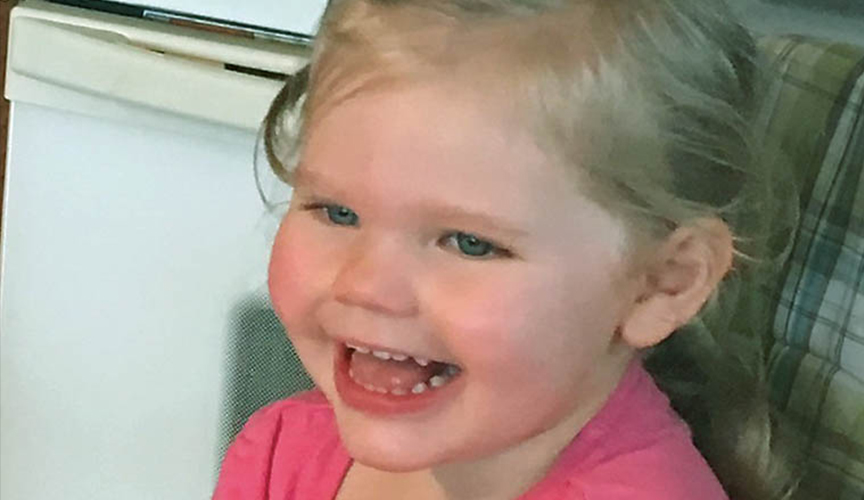 Maelee had her first seizure at 4 months old, and her seizures increased in frequency as she aged. At her worst period, Peters-Larson said her daughter would suffer from hundreds of seizures a day, including tonic-clonic, myoclonic and grand mal seizures. Anti-seizure medications failed to slow her seizure activity and changing her diet didn’t help either, said Peters-Larson. In 2016, neurologists near their Tulsa, Okla., home implanted a vagus nerve stimulator (VNS) but the device did little to reduce the amount of seizures.
Maelee had her first seizure at 4 months old, and her seizures increased in frequency as she aged. At her worst period, Peters-Larson said her daughter would suffer from hundreds of seizures a day, including tonic-clonic, myoclonic and grand mal seizures. Anti-seizure medications failed to slow her seizure activity and changing her diet didn’t help either, said Peters-Larson. In 2016, neurologists near their Tulsa, Okla., home implanted a vagus nerve stimulator (VNS) but the device did little to reduce the amount of seizures.
After running out of treatment options in Oklahoma, the Peters-Larson family then turned Le Bonheur for help. When Neurologist Stephen Fulton, MD, suggested that Maelee enroll in the hospital’s new fenfluramine drug trial, Peters-Larson signed up.
“We were excited about enrolling because nothing else was helping,” Peters-Larson said. “We needed to try something else.”
After adjusting the dosage of her new medication, Maelee was seizure free for 31 days – her longest stretch ever. Maelee’s cognitive abilities also have improved.
“Before she started taking (fenfluramine), she never had a day without a seizure,” Peters-Larson said. “Without that constant electrical storm in her brain, she can now learn and retain information.”
In addition to taking fenfluramine, patients enrolled in the trial must also continue to take their anti-seizure medications and are required to return to Le Bonheur for monthly study visits.
Fenfluramine trial at a glance:
- Phase two trial
- Treatment for seizures associated with Dravet Syndrome
- Early results show fewer seizures
- Shown to improve attention, behavior, learning comprehension and language development
Help us provide the best care for kids.
Le Bonheur Children's Hospital depends on the generosity of friends like you to help us serve 250,000 children each year, regardless of their family’s ability to pay. Every gift helps us improve the lives of children.
Donate Now