Clinical Trial: Nitric Oxide and Congenital Diaphragmatic Hernia
Tim Jancelewicz, MD, MA, MS, interim division chief of pediatric surgery at Le Bonheur Children’s Hospital and professor at the University of Tennessee Health Science Center (UTHSC), is a co-principal investigator on an $11.4 million, multi-institutional clinical trial grant to study the efficacy of the longstanding practice of using nitric oxide to treat newborns with congenital diaphragmatic hernia (CDH), a life-threatening condition that occurs when the diaphragm fails to fully develop.
Tim Jancelewicz, MD, MA, MS, is a co-principal investigator on an $11.4 million, multi-institutional clinical trial grant to study the efficacy of the longstanding practice of using nitric oxide in newborns with congenital diaphragmatic hernia (CDH). Jancelewicz is recognized as one of the world’s experts in CDH.
Jancelewicz is partnering with Matthew Harting, MD, MS, associate professor in the Department of Pediatric Surgery at McGovern Medical School at the University of Texas Health Science Center at Houston, along with a team of investigators in 19 partnering institutions, on the innovative, seven-year NoNO Trial, funded by the National Heart, Lung and Blood Institute of the National Institutes of Health. The two surgeons have studied CDH for more than a decade.
Under Jancelewicz’s direction, UTHSC and Le Bonheur will serve as the clinical coordinating center for the 19 institutions involved in the trial. Harting will direct the data coordinating center at the University of Texas Health Science Center.
“Congenital diaphragmatic hernia is a relatively rare congenital condition where there's a hole in the diaphragm. This means the child’s organs are up in the chest, and they have underdeveloped lungs and pulmonary hypertension,” Jancelewicz said. The condition affects one in 2,000 to one in 4,000 live births. “You can use inhaled nitric oxide to at least temporarily improve oxygenation in a newborn with this issue. Some people believe this can decrease the potential need for invasive care, including extracorporeal life support or ECMO, which is heart-lung bypass, the most invasive intervention.”
Historically, it has been believed that nitric oxide works to improve oxygenation enough that you might not need ECMO.
“If anything, that evidence suggests there may be a survival disadvantage to use of nitric oxide, but people are still using it, despite the lack of evidence supporting its use,” Jancelewicz continued.
The use of nitric oxide will be randomized at participating centers. In this way, every center will serve as its own control.
“For a certain period of time, they will be using nitric oxide as they always have, and then they will be randomized at some time point to stop using nitric oxide,” he said. “The only difference in care will be that nitric oxide is removed, and you'll be able to compare outcomes before and after de-implementation at each center.”

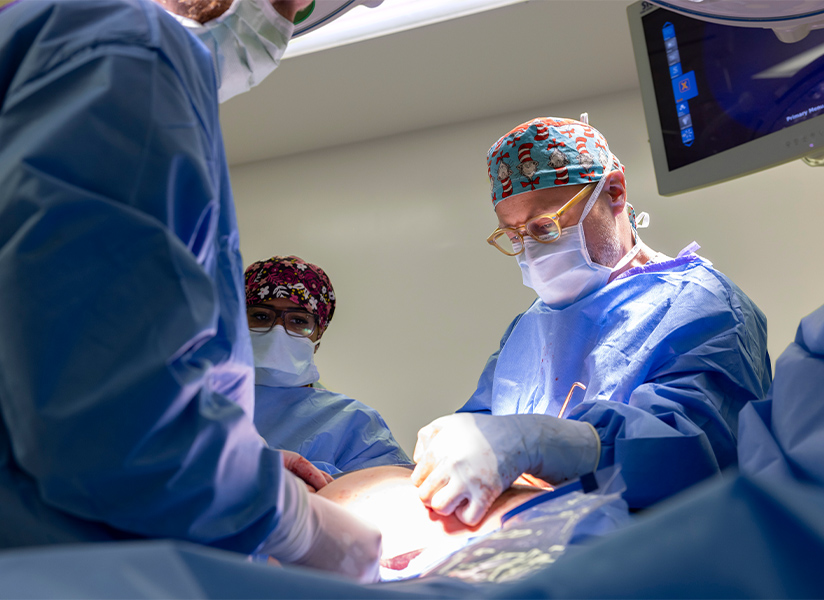
Jancelewicz, pictured above right, has studied congenital diaphragmatic hernia (CDH) for more than a decade and will direct the clinical coordinating center for the trial at Le Bonheur and the University of Tennessee Health Science Center.
The trial will also include interviews with physicians to understand barriers to de-implementation of nitric oxide and reasons for using nitric oxide to treat these newborns.
“The heart of this trial is really the design to try and overcome historic barriers in CDH research and answer some key patient management questions,” Jancelewicz said. “We tried to pick an easy question like nitric oxide. Turns out, it's not so easy. It's actually extremely complicated. If we get an answer for this question, we can then ask other questions using this approach in other neonatal diseases, where the care is very complex.”
"Dr. Jancelewicz is recognized as one of the world’s experts in the complexities of patients who are diagnosed with CDH, which is affirmed by this NIH grant award,” said Le Bonheur President and Surgeon-in-Chief Trey Eubanks, MD, FACS. “It is another testament to the excellence right here at Le Bonheur and UTHSC. We are working every day to help children and to develop future treatments that will save lives.”
Le Bonheur is among the 19 centers participating in the trial. Some of the others participating are the University of Alabama at Birmingham, Emory University, Harvard University, Arkansas Children's Research Institute, the University of Michigan, Stanford University and Vanderbilt University.
Help us provide the best care for kids.
Le Bonheur Children's Hospital depends on the generosity of friends like you to help us serve 250,000 children each year, regardless of their family’s ability to pay. Every gift helps us improve the lives of children.
Donate Now